Ensuring the safety of blood products before transfusion is paramount to preventing adverse reactions and protecting the health of veterinary patients. Veterinary blood banks should have protocols in place to establish the safety and quality of blood products obtained from donors. Examples include protocols related to donor screening and selection, blood collection, processing, storage, and handling of blood components. Quality control and assurance practices are also required to establish safety, purity, and potency of blood products.
Despite the protocols established by veterinary blood banks to safeguard the quality of blood products, factors such as improper processing, transportation, storage conditions within hospitals, and mishandling of blood products can introduce changes that compromise the safety of these products. Therefore, before transfusion, it is crucial to conduct a thorough visual inspection of blood components to mitigate the risk of adverse reactions. Several visual changes can occur in blood components that may indicate potential issues with the product’s safety or quality.
Color Change of RBC Units
Color change in red blood cells (RBCs) and plasma components can indicate hemolysis, oxidation, bacterial contamination, or improper storage conditions that could pose a risk for patients. Hemolyzed blood products carry significant risks such as acute hemolytic transfusion reactions (AHTR), renal dysfunction, thromboembolic events, inflammatory responses, and transfusions-associated circulatory overload (TACO). Transfusing an oxidized component includes risks of oxidative stress, inflammatory response, immunomodulation, endothelial and organ dysfunction, and thromboembolic events. Bacterial contamination of blood products can lead to sepsis or other serious complications if transfused.
RBC units can demonstrate various color changes indicating different issues with the quality and integrity of the product. Darkening or brownish color change can indicate oxidation of hemoglobin due to exposure to air, light, or other oxidizing agents during processing, storage, or transportation (Figure 1). Darkening in RBC units can also be an indication of clot formation or aggregation, which could lead to occlusion of blood vessels if transfused (Figure 2). Cloudiness or turbidity of an RBC unit may suggest bacterial contamination or lipid accumulation, whereas a yellowish color can indicate hyperbilirubinemia.
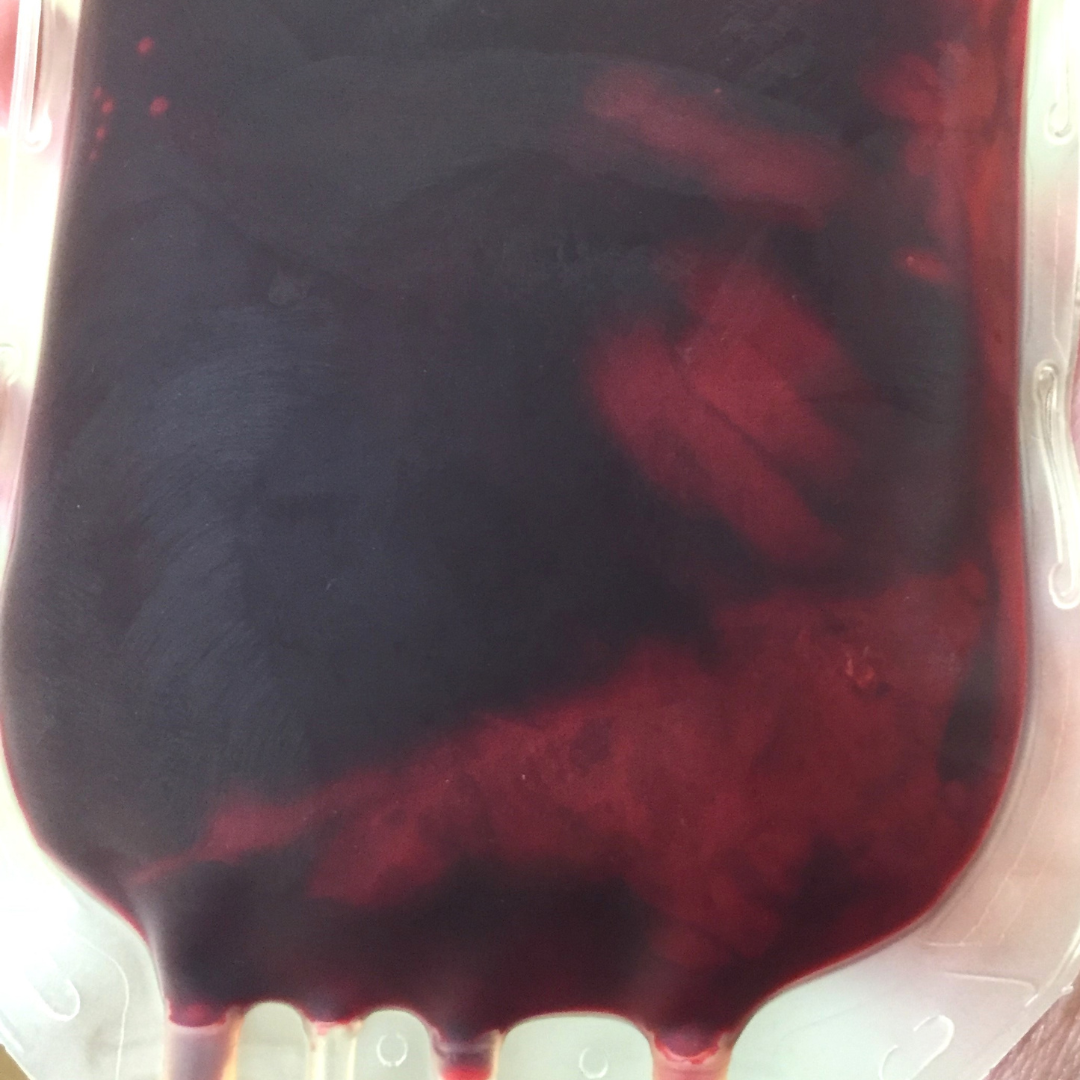
Figure 1: Darkening color change of an RBC unit can occur due to exposure to air, light, or other oxidizing agents during processing, storage, or transportation.
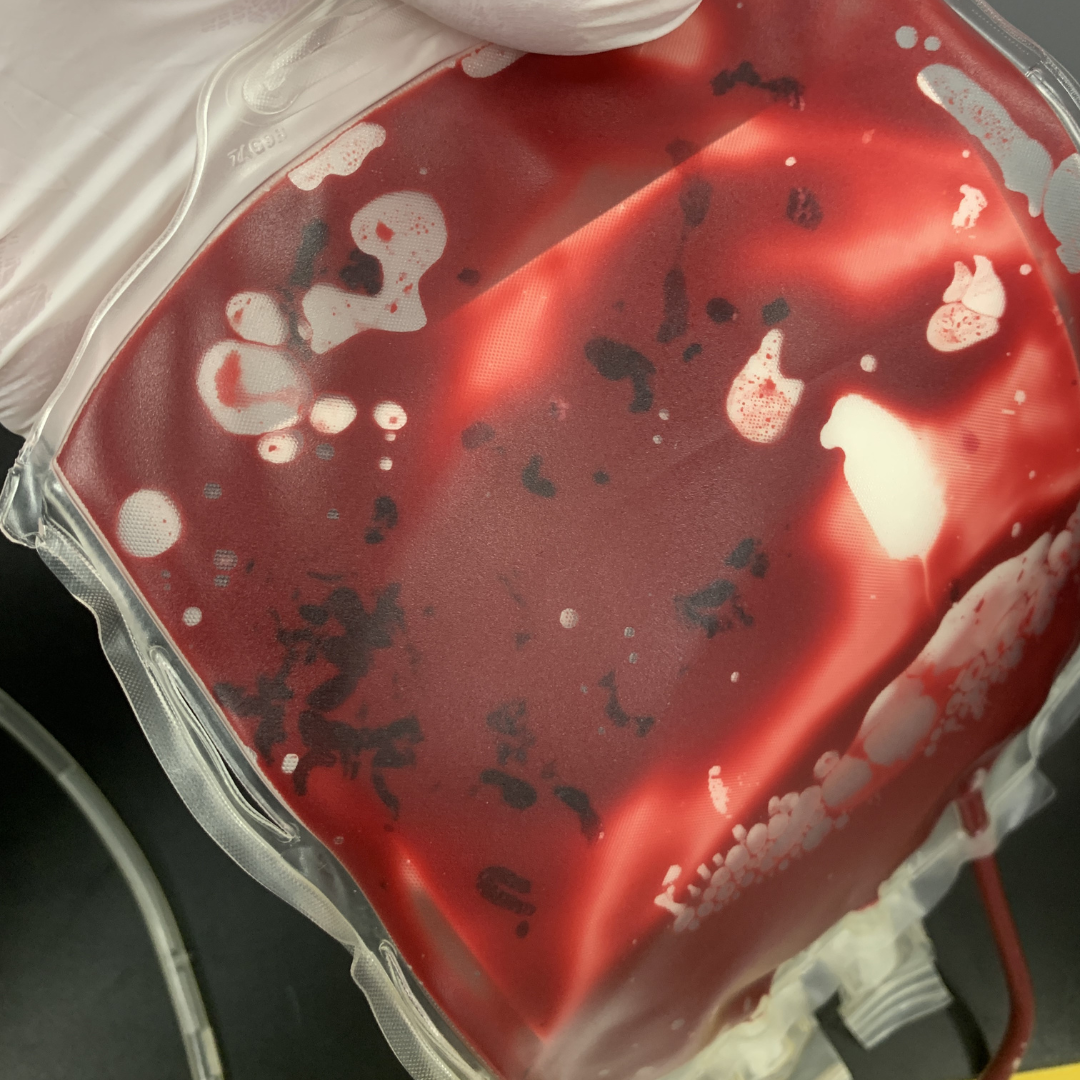
Figure 2: The presence of clots in an RBC unit can lead to occlusion of vessels if transfused.
Color Change of Plasma Units
Color changes in plasma units include excessive yellowing, pink or reddish tint, cloudiness, or a milky white hue. Severely yellow plasma units can indicate hyperbilirubinemia while a pink or reddish coloration could indicate hemolysis (Figure 3). Cloudy or turbid plasma units can indicate bacterial contamination or lipid accumulation. White or milky plasma appearance might indicate the presence of lipid or protein aggregates due to improper storage conditions, temperature fluctuations, or processing techniques (Figure 4).
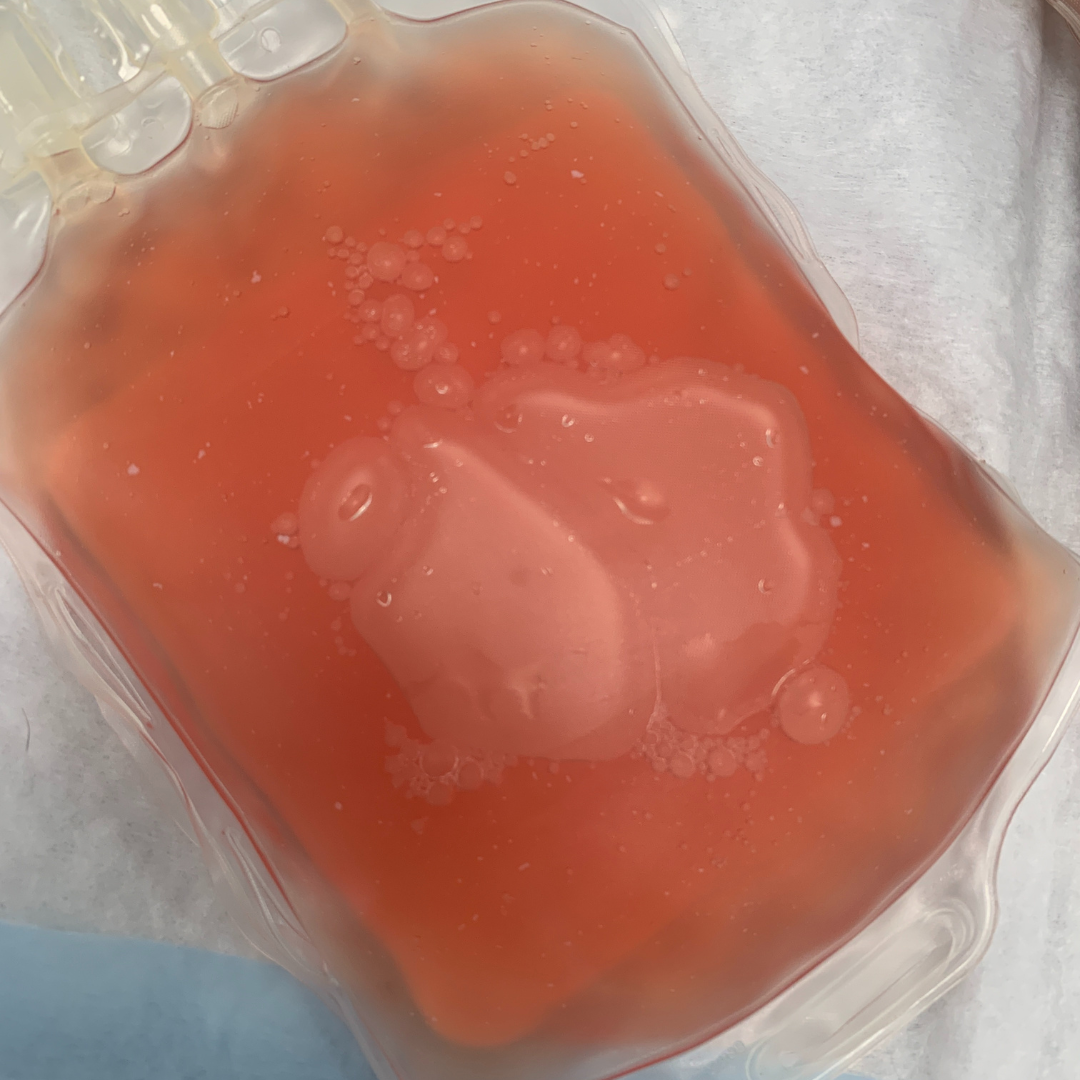
Figure 3: A red-tinged plasma unit can indicate hemolysis.

Figure 4: A milky plasma unit can indicate the presence of lipid or protein aggregates due to improper storage conditions, temperature fluctuations, or processing techniques.
Gas Formation in Units
Gas formation in blood components, characterized by the presence of gas bubbles or foaming, can indicate bacterial contamination or improper storage conditions such as exposure to air or inadequate sealing of the blood bag (Figure 5).
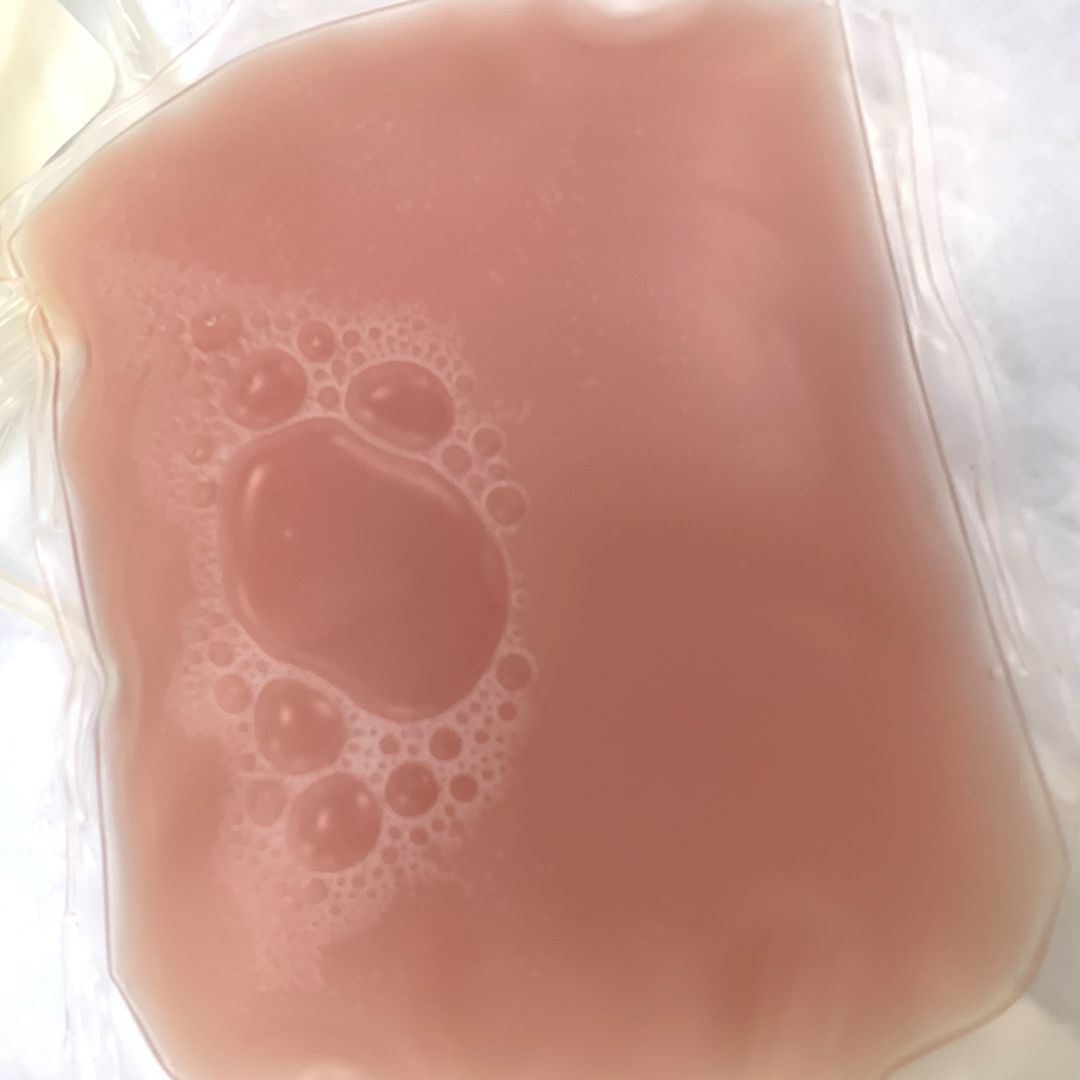
Figure 5: Gas bubbles in a plasma unit can indicate bacterial contamination or improper storage conditions such as exposure to air or inadequate sealing of the blood bag.
Particulate Matter in Units
Sometimes, units can show the presence of particulate matter, characterized by white spots or foreign material suspended in the blood component. This can indicate contamination or degradation of the product. Visual inspection of plasma units can reveal fibrin formation, where fibrin strands or clots may be visible within the plasma. Fibrin formation can occur due to improper handling, processing or storage conditions. Excessive fibrin in plasma can be potentially harmful if transfused, leading to thromboembolic events, vascular occlusion, acute transfusion reactions, or, in rare cases, disseminated intravascular coagulation.
White particulate matter (WPM) may be present in plasma or RBC units (Figure 6). WPM is composed of normal blood cell elements, namely aggregates of platelets with variable amounts of fibrin and trapped red or white blood cells. The exact cause of WPM is unclear, but factors such as high platelet count, lipemia, or use of hard spin during processing may contribute to its formation. It is not definitively known if the presence of WPM causes adverse effects but caution should be used before transfusing the unit given the potential risks.
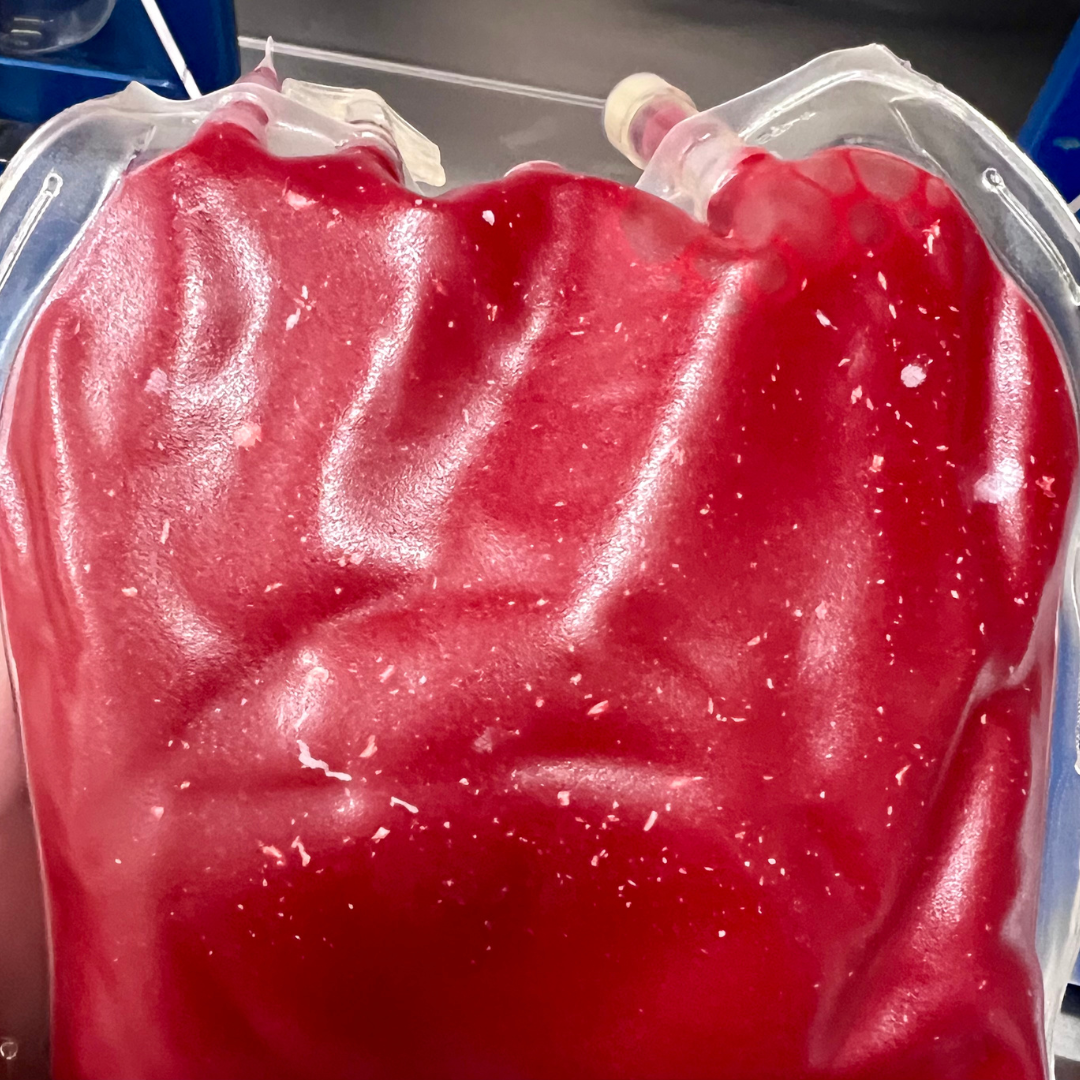
Figure 6: White particulate matter in an RBC unit can form due to high platelet count, lipemia, or use of hard spin during processing.
Conclusion
Ensuring the safety of blood products before transfusions is crucial for safeguarding the health of veterinary patients. While veterinary blood banks have protocols in place to establish the safety and quality of blood products, visual inspection plays a vital role in identifying potential issues prior to transfusion. It is essential for veterinary professionals to remain vigilant and exercise caution before transfusing blood products to mitigate the risk of adverse reactions and protect the wellbeing of their patients.
Written by Jessie Brown, BS, LVT who is a volunteer member of the Canadian Animal Blood Bank Education Advisory Committee and Blood Bank Director for the Veterinary Emergency Group.


